2025 was a busy year at the FDA’s oncology desk. More than 50 cancer-related approvals came through, and while I can’t cover them all here, I want to walk through some of the most significant ones – the drugs that represent new therapeutic classes, address unmet needs, or mark a shift in how we approach certain cancers. I’ve organized this by quarter, with the relevant biomarkers and mechanisms for each.
A Quick Primer on the Major Drug Classes
Before diving into specific approvals, it’s worth understanding the major therapeutic platforms that shaped the 2025 landscape: antibody-drug conjugates, bispecific antibodies, checkpoint inhibitors, and small molecule kinase inhibitors.
Antibody-Drug Conjugates
ADCs have been around for years, but the technology has matured considerably. The concept is elegant: take a monoclonal antibody that recognizes a tumor-associated antigen, attach a cytotoxic payload via a chemical linker, and let the antibody deliver the chemotherapy directly to the cancer cell. The antibody binds its target, the complex gets internalized, and the payload is released inside the cell.
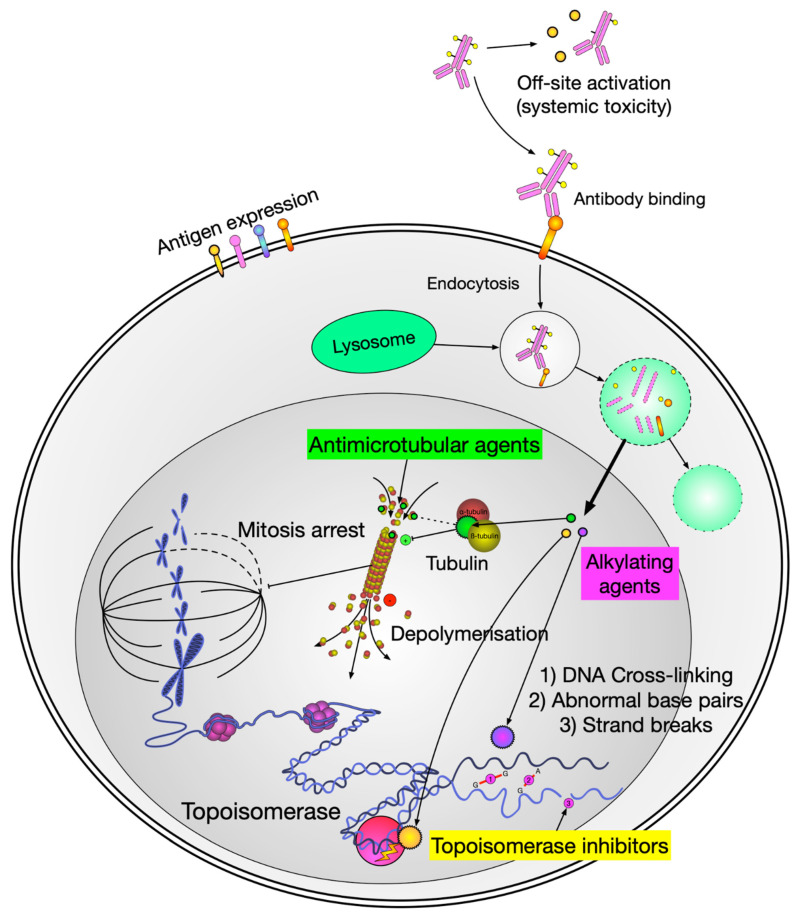
The appeal is targeting – you’re delivering a highly potent drug specifically to cells expressing the target antigen. The bystander effect (payload diffusing to nearby cells) can actually be helpful in solid tumors with heterogeneous antigen expression.
Bispecific Antibodies
Bispecifics are engineered to bind two different targets simultaneously. In oncology, the most common design connects a tumor-associated antigen with CD3 on T cells, physically bridging the immune effector to the cancer cell. This forces an immunological synapse regardless of whether the T cell would normally recognize that tumor.
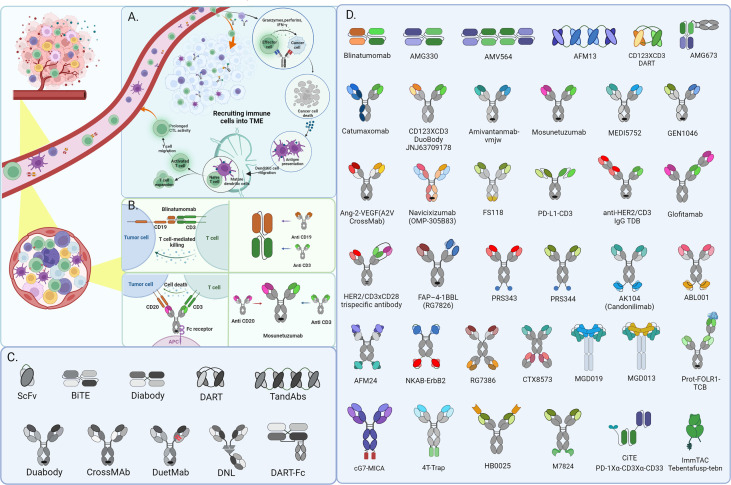
The downside is cytokine release syndrome (CRS) and neurologic toxicity – the same issues we see with CAR-T cells. These drugs require step-up dosing and close monitoring, at least initially.
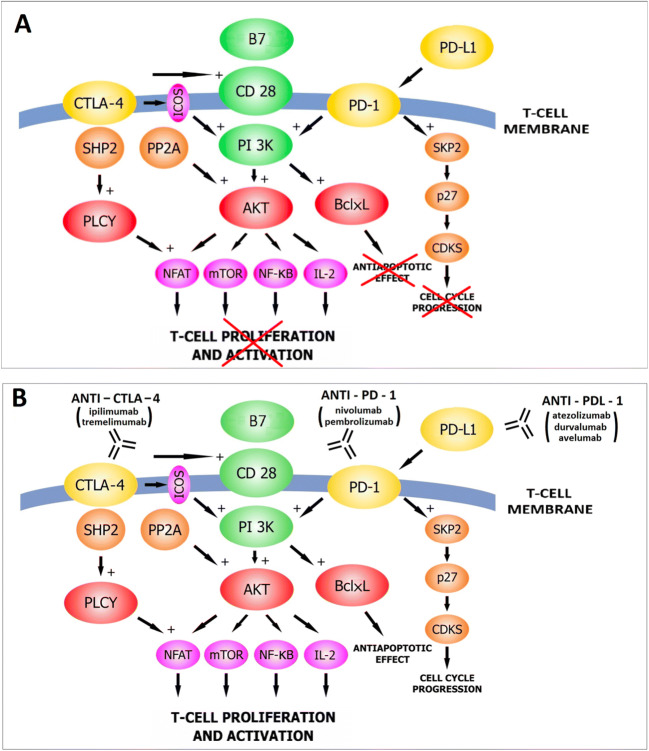
Checkpoint Inhibitors
Several 2025 approvals expanded the use of pembrolizumab and durvalumab into new settings. I won’t belabor the mechanism – by now most readers know that anti-PD-1 and anti-PD-L1 antibodies release the brakes on T cells – but the perioperative applications are worth noting.
Small Molecule Kinase Inhibitors
The workhorses of targeted therapy remain the small molecule inhibitors – oral drugs that slip into the ATP-binding pocket of kinases and block their activity. With nearly 70 FDA-approved kinase inhibitors for cancer as of 2024, this is a mature drug class, but new entries keep coming, often with improved selectivity or activity against resistant mutations.
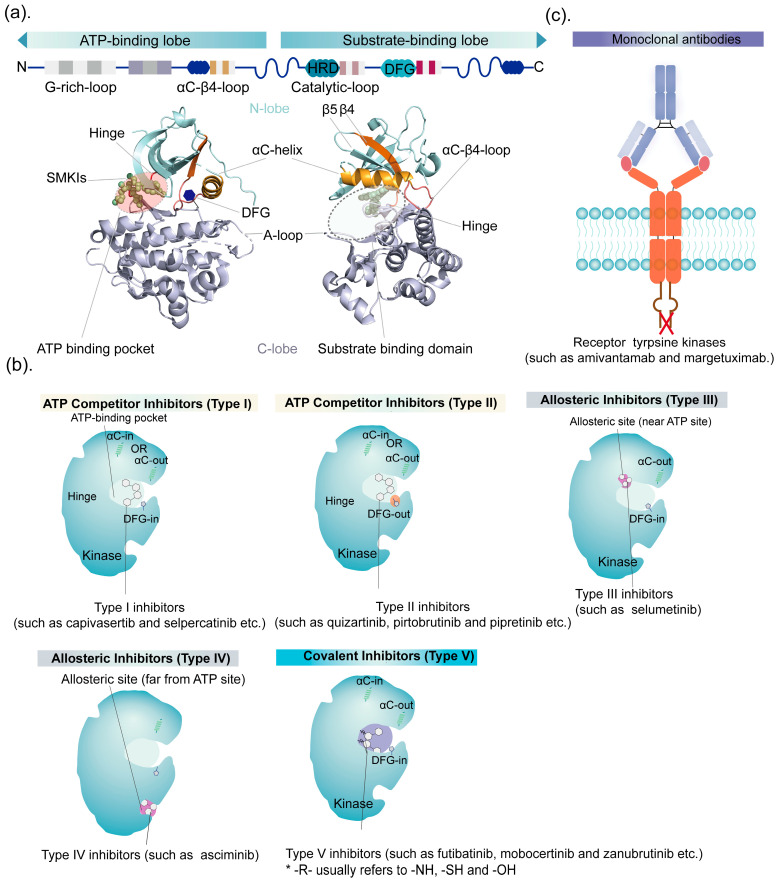
The distinction between inhibitor types matters clinically. Type I inhibitors (like erlotinib, gefitinib) compete directly with ATP in the active kinase conformation. Type II inhibitors (like imatinib, sorafenib) trap the kinase in an inactive “DFG-out” state, often providing better selectivity. The newer generation includes allosteric inhibitors that bind outside the ATP pocket entirely, which can overcome resistance mutations that arise in the ATP-binding site.
Several 2025 approvals fall into this category: zongertinib (HER2), sunvozertinib (EGFR exon 20), imlunestrant (ESR1), ziftomenib (menin), mirdametinib and selumetinib (MEK), vimseltinib (CSF1R), and belzutifan (HIF-2α). The trend is toward increasing selectivity – sparing related kinases to reduce off-target toxicities – and designing around known resistance mechanisms from the start.
Q1 2025
Datopotamab Deruxtecan (Datroway) – HR+/HER2- Breast Cancer
| Approved January 17, 2025 | Daiichi Sankyo / AstraZeneca |
This TROP-2-targeting ADC joins sacituzumab govitecan in an increasingly crowded space. The payload is a topoisomerase I inhibitor (a deruxtecan, like in trastuzumab deruxtecan). In the TROPION-Breast01 trial, 732 patients with HR+/HER2- metastatic breast cancer who had progressed on endocrine therapy and at least one line of chemo were randomized to dato-DXd versus physician’s choice chemotherapy.
Median PFS was 6.9 months versus 4.9 months – modest, but this is a heavily pretreated population with limited options. The interesting question going forward is how dato-DXd and sacituzumab will be sequenced, given that they target the same antigen.
FDA Drug Trials Snapshots: DATROWAY
Mirdametinib (Gomekli) – NF1 Plexiform Neurofibromas
| Approved February 11, 2025 | SpringWorks Therapeutics |
NF1 is a tumor suppressor gene encoding neurofibromin, which negatively regulates RAS signaling. Loss of NF1 leads to constitutive RAS pathway activation, and plexiform neurofibromas are a hallmark manifestation – benign but locally aggressive nerve sheath tumors that can cause significant morbidity.
Mirdametinib is a MEK inhibitor (downstream of RAS). In the ReNeu trial, 41% of adults and 52% of children with symptomatic, inoperable plexiform neurofibromas had confirmed tumor shrinkage. This follows selumetinib (approved for pediatric NF1 in 2020) and gives us another option in this space.
FDA Approves Mirdametinib for NF1
Vimseltinib (Romvimza) – Tenosynovial Giant Cell Tumor
| Approved February 14, 2025 | Deciphera Pharmaceuticals |
TGCT is a rare condition where the joint lining develops a locally aggressive tumor. It’s driven by a chromosomal translocation that creates a COL6A3-CSF1 fusion, leading to CSF1 overexpression and recruitment of macrophages that cause the tumor to swell and damage the joint.
Vimseltinib inhibits CSF1R. In the pivotal trial, 40% of patients had tumor responses versus 0% on placebo, with improvements in joint function and pain. For patients with TGCT where surgery would cause unacceptable morbidity, this provides a medical alternative.
FDA Drug Trials Snapshot: ROMVIMZA
Tislelizumab (Tevimbra) – Esophageal Squamous Cell Carcinoma
| March 2025 (Label Expansion) | BeiGene |
Tislelizumab is a PD-1 inhibitor that got its initial US approval for second-line ESCC in 2024. The 2025 expansion adds first-line use in combination with platinum-based chemotherapy for PD-L1+ (CPS ≥1) unresectable or metastatic ESCC.
The RATIONALE-302 data showed improvement in median OS from 6.3 to 8.6 months in the second-line setting. Moving to first-line is the natural progression for checkpoint inhibitors that show activity in later lines.
FDA Drug Trials Snapshots: TEVIMBRA
Durvalumab (Imfinzi) – Muscle-Invasive Bladder Cancer
| Approved March 28, 2025 | AstraZeneca |
This is a perioperative indication – durvalumab combined with neoadjuvant chemotherapy before cystectomy, then continued as adjuvant monotherapy afterward. The NIAGARA trial enrolled 1,063 patients with muscle-invasive bladder cancer.
The idea of “bookending” surgery with immunotherapy makes mechanistic sense: shrink the tumor and prime an immune response before resection, then consolidate after surgery to mop up residual disease. Event-free survival was significantly improved compared to chemotherapy and surgery alone. This is the first perioperative checkpoint inhibitor approval in bladder cancer.
FDA Approves Durvalumab for Muscle Invasive Bladder Cancer
Q2 2025
Avutometinib + Defactinib – Low-Grade Serous Ovarian Cancer
| Approved April 28, 2025 (Accelerated) | Verastem |
This is a notable one. Low-grade serous ovarian cancer accounts for only 2-5% of ovarian cancers, tends to occur in younger women, and is notoriously chemoresistant. It’s a different disease from high-grade serous – different mutations (often KRAS, BRAF, or NRAS), different behavior, different biology.
Avutometinib is a MEK inhibitor; defactinib inhibits FAK. The rationale for the combination is that FAK activation is a common mechanism of resistance to MEK inhibition – so you hit both pathways simultaneously. In the RAMP 201 trial, the combination achieved a 44% response rate and 22-month median PFS in KRAS-mutated patients.
This is the first FDA-approved treatment specifically for LGSOC. For a disease with essentially no approved targeted therapies until now, that’s significant.
FDA Drug Trials Snapshot: AVMAPKI FAKZYNJA CO-PACK
Belzutifan (Welireg) – Pheochromocytoma and Paraganglioma
| Approved May 14, 2025 | Merck |
Pheochromocytomas and paragangliomas (collectively PPGL) are rare neuroendocrine tumors that secrete catecholamines, causing hypertension, headaches, and other symptoms. Many harbor germline mutations in succinate dehydrogenase subunits (SDHx), creating a pseudohypoxic state with HIF accumulation.
Belzutifan inhibits HIF-2α directly. It was already approved for VHL-associated renal cell carcinoma (VHL disease also causes HIF stabilization via a different mechanism). In PPGL, the LITESPARK-015 trial showed a 26% response rate with a median duration of response over 20 months.
This is the first oral systemic therapy approved for PPGL. Previously, treatment options were limited to surgery, radiation, and high-dose 131I-MIBG for selected patients.
FDA Approves Belzutifan for Pheochromocytoma or Paraganglioma
Telisotuzumab Vedotin (Emrelis) – c-Met-Overexpressing Lung Cancer
| Approved May 14, 2025 (Accelerated) | AbbVie |
c-Met overexpression occurs in a subset of NSCLC and is associated with poor prognosis. This ADC targets c-Met with an MMAE payload (same payload as brentuximab and polatuzumab). The indication requires high c-Met expression (≥50% of tumor cells with 3+ IHC staining).
This is the first c-Met-directed ADC to gain approval. Whether it will find a niche alongside the c-Met TKIs (capmatinib, tepotinib for MET exon 14 skipping) remains to be seen – those are driven by activating mutations rather than overexpression, so the patient populations don’t fully overlap.
FDA Approval Package for Emrelis
Pembrolizumab (Keytruda) – Perioperative Head and Neck Cancer
| Approved June 12, 2025 | Merck |
This is the first perioperative anti-PD-1 regimen for head and neck squamous cell carcinoma. In KEYNOTE-689, pembrolizumab was given neoadjuvantly with surgery, then adjuvantly with radiation therapy, then continued as monotherapy. Patients had resectable locally advanced HNSCC with PD-L1 CPS ≥1.
The result: a 30% reduction in the risk of event (recurrence, progression, or death). Median event-free survival was 59.7 months versus 29.6 months. That’s a substantial improvement in a disease where local-regional recurrence has historically been difficult to prevent.
The perioperative approach is gaining traction across tumor types – bladder, head and neck, and likely more to come.
FDA Oncology Approval Notifications
Q3 2025
Sunvozertinib (Zegfrovy) – EGFR Exon 20 Insertion Lung Cancer
| Approved July 2, 2025 (Accelerated) | Dizal Pharmaceuticals |
EGFR exon 20 insertion mutations have been a therapeutic challenge. Unlike the classic EGFR-sensitizing mutations (exon 19 deletions, L858R), these insertions create conformational changes that standard EGFR TKIs don’t effectively target. Patients with these mutations were essentially left behind while EGFR-mutant NSCLC became a model of precision oncology.
Sunvozertinib was designed specifically for exon 20 insertions. In the WU-KONG1B trial, the response rate was 46% with a median duration of 11.1 months – numbers that would be unremarkable for classic EGFR mutations but are impressive for this subset.
The Oncomine Dx Express Test was approved as a companion diagnostic alongside the drug.
FDA Grants Accelerated Approval to Sunvozertinib
Linvoseltamab (Lynozyfic) – Multiple Myeloma
| Approved July 2, 2025 (Accelerated) | Regeneron |
Another BCMA-CD3 bispecific, joining teclistamab and elranatamab. In myeloma, BCMA is nearly ubiquitously expressed on plasma cells, making it an attractive target for both bispecifics and CAR-T cells.
In LINKER-MM1, 70% of heavily pretreated patients (median 5 prior lines) responded, with 50% achieving complete response or better. Responses were durable – 72% maintained their response at 12 months. The main concerns are the usual bispecific toxicities: CRS (40%) and ICANS (6%), necessitating hospitalization for the first doses.
The field is now debating how to sequence these agents – bispecifics versus CAR-T, and which bispecific when multiple options exist.
FDA Grants Accelerated Approval to Linvoseltamab
Zongertinib (Hernexeos) – HER2-Mutant Lung Cancer
| Approved August 8, 2025 (Accelerated) | Boehringer Ingelheim |
HER2 mutations in NSCLC (distinct from HER2 amplification or overexpression) occur in roughly 2-4% of cases and are enriched in never-smokers. Trastuzumab deruxtecan received accelerated approval for this indication in 2022, but there’s room for oral options.
Zongertinib is a highly selective HER2 TKI, designed to spare EGFR (whose inhibition causes rash and diarrhea). In Beamion LUNG-1, 75% of patients without prior HER2-targeted therapy responded. That’s a striking number, and the selectivity profile may mean better tolerability than agents that hit both HER2 and EGFR.
FDA Drug Trials Snapshots: HERNEXEOS
Imlunestrant (Inluriyo) – ESR1-Mutated Breast Cancer
| Approved September 25, 2025 | Eli Lilly |
ESR1 mutations are a major mechanism of resistance to aromatase inhibitors in ER+ breast cancer. These mutations constitutively activate the estrogen receptor even in the absence of estrogen, rendering standard endocrine therapies ineffective.
Imlunestrant is an oral SERD (selective estrogen receptor degrader). Unlike fulvestrant, which requires intramuscular injections, imlunestrant is taken by mouth. In the EMBER-3 trial, it reduced the risk of progression or death by 38% in patients with ESR1 mutations compared to standard therapy.
Notably, the Guardant360 CDx liquid biopsy was approved as a companion diagnostic. ESR1 mutations often emerge under treatment pressure and may be more readily detected in circulating tumor DNA than in tissue biopsies from the primary tumor.
FDA Approval Package for Inluriyo
Q4 2025
Tarlatamab (Imdelltra) – Small Cell Lung Cancer
| Traditional Approval November 2025 | Amgen |
Tarlatamab first received accelerated approval in May 2024 based on response rate data. The 2025 traditional approval came after the DeLLphi-304 trial demonstrated overall survival benefit: median OS of 13.6 months versus 8.3 months with chemotherapy, a 40% reduction in the risk of death.
Small cell lung cancer is an aggressive disease with dismal outcomes after first-line therapy – median OS has historically been around 6-7 months. Tarlatamab targets DLL3, a protein expressed on most SCLC cells but largely absent from normal adult tissues, and engages T cells via CD3.
The boxed warning for CRS and neurologic toxicity means this requires careful monitoring, but for patients with limited options, the survival benefit is meaningful.
FDA Approval Announcement for Tarlatamab
Ziftomenib (Komzifti) – NPM1-Mutated AML
| Approved November 13, 2025 | Kura Oncology |
This is a genuinely exciting approval. NPM1 mutations are the most common genetic alteration in AML, found in about 30% of cases. They cause aberrant cytoplasmic localization of nucleophosmin and create a dependency on the menin-MLL interaction for maintaining the leukemic state.
Ziftomenib is a menin inhibitor – the first in its class to receive approval. In KO-MEN-001, it induced remissions in relapsed/refractory NPM1-mutated AML, a population with very poor prognosis.
The main safety concern is differentiation syndrome (26% of patients), which occurs when leukemia cells rapidly mature in response to therapy. It’s the same phenomenon seen with ATRA in acute promyelocytic leukemia, and it requires vigilant monitoring and prompt treatment with steroids.
FDA Approves Ziftomenib for AML with NPM1 Mutation
Epcoritamab + Lenalidomide/Rituximab – Follicular Lymphoma
| Approved November 18, 2025 | Genmab / AbbVie |
Epcoritamab is a CD20-CD3 bispecific that was already approved for large B-cell lymphoma. This approval adds follicular lymphoma in combination with lenalidomide and rituximab – a triple combination that layers bispecific T-cell engagement on top of conventional immunochemotherapy.
In EPCORE FL-1, the combination reduced the risk of progression or death by 79% compared to lenalidomide plus rituximab alone. The 89% overall response rate is impressive even for a relatively indolent lymphoma.
As with other bispecifics, the label carries boxed warnings for CRS and ICANS. But the efficacy signal is strong enough that this will likely become a standard option for relapsed follicular lymphoma.
Epkinly Prescribing Information (2025)
Selumetinib (Koselugo) – NF1 Plexiform Neurofibromas in Adults
| November 2025 | AstraZeneca |
Selumetinib was approved for pediatric NF1 in 2020 based on the SPRINT trial. The 2025 expansion adds adult patients with symptomatic, inoperable plexiform neurofibromas. In the KOMET trial, 20% of adults on selumetinib had confirmed responses versus 5% on placebo, with improvements in pain scores.
This provides continuity of care for NF1 patients as they age out of pediatric oncology – an increasingly important consideration as we develop more treatments for hereditary cancer predisposition syndromes.
FDA Oncology Approval Notifications
What 2025 Tells Us
A few themes stand out:
Biomarker selection is now expected. Nearly every approval above requires a test – a mutation, a protein expression level, a pathway activation status – before treatment. We’re well past the era of empiric chemotherapy for most cancers. The infrastructure for molecular profiling needs to keep pace with drug development.
The perioperative setting is opening up. Durvalumab in bladder cancer, pembrolizumab in head and neck cancer – these represent a conceptual shift from treating advanced disease to preventing recurrence around the time of curative-intent surgery. If these approaches continue to show benefit, we may see immunotherapy move earlier into the treatment course for many tumor types.
Bispecifics are maturing. We now have multiple BCMA-directed bispecifics in myeloma, CD20-directed bispecifics in lymphoma, and DLL3-directed tarlatamab in SCLC. The toxicity profiles are becoming predictable, and we’re developing experience with step-up dosing and CRS management.
Rare tumors are no longer neglected. LGSOC, PPGL, TGCT – each of these had essentially no approved targeted therapies until this year. The orphan drug pathway and accelerated approval are working as intended, getting treatments to patients with diseases too rare for traditional development timelines.
Liquid biopsy is gaining ground. The Guardant360 approval with imlunestrant highlights the utility of detecting resistance mutations from blood rather than requiring repeat tissue biopsies. This is particularly valuable for acquired resistance mechanisms that may not be present in the primary tumor.
Further Reading
- FDA Oncology Approval Notifications (Complete List)
- AACR: FDA Approvals in Oncology, January–March 2025
- AACR: FDA Approvals in Oncology, April–June 2025
- AACR: FDA Approvals in Oncology, July–September 2025
- AACR: FDA Approvals in Oncology, October–December 2025
- CancerNetwork: Top 10 FDA Oncology Approvals in 2025
- ASCO Post: New FDA-Approved Oncology Drugs in 2025
- MSK: Research Behind 2025 FDA Cancer Drug Approvals
This is an educational overview, not medical advice. Treatment decisions should be made with your oncologist based on your individual situation.
Comments